The Zequanox Story
The naturally occurring microorganism in Zequanox (a bacterium that kills invasive zebra and quagga mussels but leaves other mollusks unharmed) was discovered by the New York State Museum.
Marrone Bio Innovations commercialized the product Zequanox. More recently, Invasive Species Corporation, co-founded by the founder and former CEO and former president/CFO of Marrone Bio, has taken over Zequanox to continue to expand its use and develop an improved, lower cost product for larger facilities and water bodies.
Introduction
 They are tiny—the size of a person’s fingernail—yet they are having a billion-dollar impact on the North American economy and causing great harm to countless freshwater ecosystems. Zebra mussels (Dreissena polymorpha) and quagga mussels (Dreissena rostriformis bugensis) are highly invasive bivalves native to Eastern Europe. These tiny, freshwater species originated in the Caspian and Black Seas and were transported to North America in ballast water from a cargo ship. First discovered in the Great Lakes in the late 1980s, these mussels have now invaded waterways across the United States, causing numerous ecological impacts and creating operations and maintenance challenges for commercial facilities that draw water from infested lakes and rivers. By the early 1990s facility operators were employing a variety of methods to try and stave off impending damage that could be caused by the ever-expanding mussel colonies. Some methods were labor intensive, some turned out to be completely ineffective and others posed a tremendous risk to the facilities’ employees and the surrounding ecosystem. Without a better alternative, operators had to tolerate these shortcomings. In 2007, however, an exciting scientific breakthrough led to the development of Zequanox®, a naturally derived molluscicide that offered a highly effective AND environmentally compatible control method for these invasive mussels. Presented herein is the story of Zequanox: how it was discovered, how the product was developed, and an overview of the science behind this innovation. This document also summarizes results that demonstrate the efficacy and safety of Zequanox and highlights the product’s many advantages over traditional control options.
They are tiny—the size of a person’s fingernail—yet they are having a billion-dollar impact on the North American economy and causing great harm to countless freshwater ecosystems. Zebra mussels (Dreissena polymorpha) and quagga mussels (Dreissena rostriformis bugensis) are highly invasive bivalves native to Eastern Europe. These tiny, freshwater species originated in the Caspian and Black Seas and were transported to North America in ballast water from a cargo ship. First discovered in the Great Lakes in the late 1980s, these mussels have now invaded waterways across the United States, causing numerous ecological impacts and creating operations and maintenance challenges for commercial facilities that draw water from infested lakes and rivers. By the early 1990s facility operators were employing a variety of methods to try and stave off impending damage that could be caused by the ever-expanding mussel colonies. Some methods were labor intensive, some turned out to be completely ineffective and others posed a tremendous risk to the facilities’ employees and the surrounding ecosystem. Without a better alternative, operators had to tolerate these shortcomings. In 2007, however, an exciting scientific breakthrough led to the development of Zequanox®, a naturally derived molluscicide that offered a highly effective AND environmentally compatible control method for these invasive mussels. Presented herein is the story of Zequanox: how it was discovered, how the product was developed, and an overview of the science behind this innovation. This document also summarizes results that demonstrate the efficacy and safety of Zequanox and highlights the product’s many advantages over traditional control options.
A Multibillion Dollar Problem
Zebra and quagga mussels can attach by the millions to one another, forming dense colonies in heavy masses up to a foot thick (U.S. Department of the Interior, U.S. Geological Survey [USGS] 2008). These colonies can clog piping, filters and screens, impeding or preventing the flow of critical cooling or process water. The mussels can also cover system components and mechanical parts, causing system damage and weighing down equipment and infrastructure. These effects can hinder or in some cases shut down operations. Continual attachment of Dreissena can also significantly increase corrosion rates of steel and concrete (Benson and Raikow 2011), leaving equipment and infrastructure vulnerable to failure. Many of the traditional chemical control options, chlorine in particular, exacerbate the increased corrosion and pitting that invasive mussels initiate. Once introduced into a new water body, the population growth of these mussels can be explosive.

Very successful invaders, zebra and quagga mussels thrive in a variety of temperatures, readily find food, reproduce prolifically and rapidly, and lack natural predators. The average female zebra mussel, which is ready to reproduce in its first year of life, can release 30,000 to 40,000 eggs per year. After hatching, the planktonic larvae can not only move great distances in flowing water, but can also easily invade small places in both natural systems and industrial systems that draw from infested waters. Mussel colonies can build up very quickly. It has been reported that these mussels have been able to clog a three-foot-diameter pipe in less than three months (U.S. Department of Energy, National Energy Technology Laboratory [NETL] 2006).
An added challenge is that these mussels can rapidly disperse to other water bodies, primarily by the larval movement and their inadvertent transport by barge and boat traffic, and can survive for many days out of water, factors that have caused the zebra and quagga mussel invasion to spread to many previously uninfested waters throughout the United States. They arrived in Lake Erie in the late 1980s, likely in the ballast water of transoceanic vessels (McMahon, 1996). Dreissenids can survive dry conditions for several days on or in boats, motors, and trailers. They also hitchhike on aquarium plants, such as moss balls available at petand aquarium stores (U.S. Geological Survey, 2021). Zebra mussels were the first to arrive and establish. Where both species exist, quagga mussels frequently replace zebra mussels because they are larger. Since their invasion, zebra mussels have spread to 31 states and quagga mussels to 18 states (U.S. Geological Survey, 2023). Bilge and livewell water of recreational vessels and ballast water of shipping vessels have been the primary vectors of transmission.
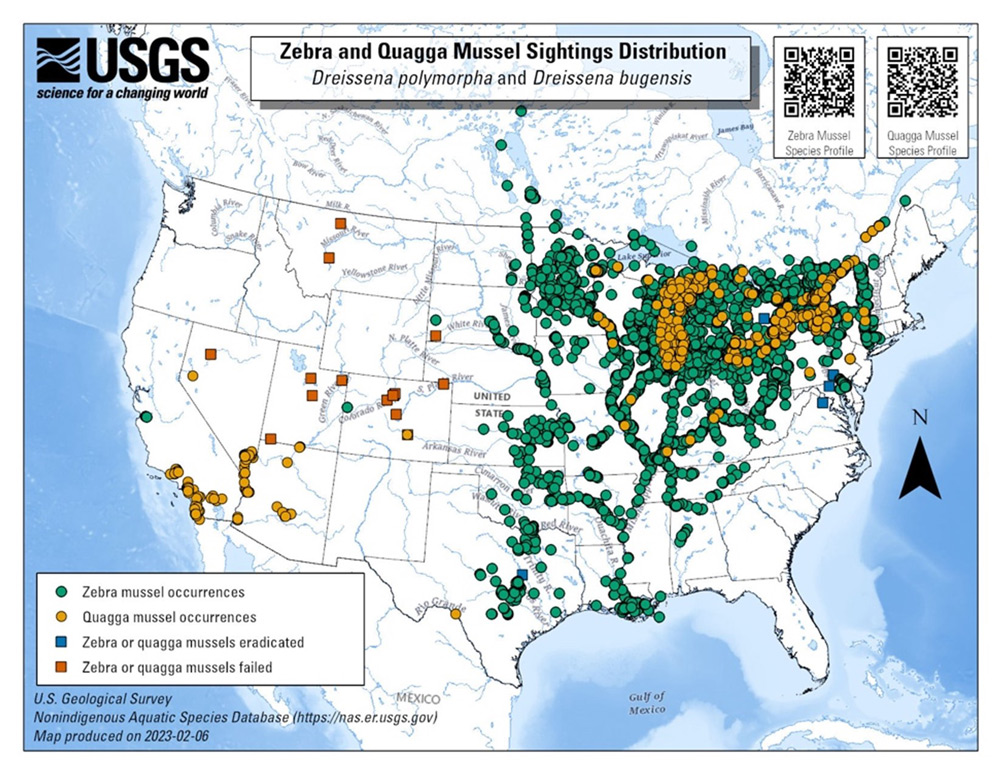
There is widespread agreement that zebra and quagga mussels annually cause millions of dollars in additional maintenance expenses in North America. United States Congressional researchers estimated that zebra mussels alone cost the power industry $3.1 billion during 1993–1999. The U.S. Fish and Wildlife Service estimated the economic impact during 2000–2010 at $5 billion. The mounting costs, combined with an ever-expanding geographical area of impact, have increased the need for reliable control methods that are suitable for a variety of industrial and civil applications.
The Need for a New Approach
Enclosed Systems Commercial and public entities facing zebra and quagga mussel infestations have applied a variety of methods when seeking to control mussel populations, including aqueous controls, antifouling coatings, physical removal and mechanical controls. Each of these methods has significant drawbacks.
 A common approach to mussel control is aqueous applications of chemicals such as chlorine for enclosed systems such as pipes and bays. Chlorine-based methods using hypochlorite, chlorine gas and chlorine dioxide necessarily involve careful practices to ensure that the chemicals are safely stored, and that employees handling the chemicals are not exposed to hazards and unnecessary risk. In addition, chlorine and other oxidizing chemicals are corrosive to equipment. Chemical treatments are toxic to other aquatic organisms and because of this non-targeted toxicity, facilities using chlorine and other chemical- based molluscicides may be required to deactivate or detoxify the treated water before discharge to meet environmental requirements (NETL 2006). Bisulfate or similar salts are used to help prevent the release of chlorine into the environment and reduce the impact on other aquatic organisms, contributing to salt loading in water bodies. Many molluscicides require the addition of clay to a treated water system to quench or deactivate the chemicals’ toxicity before discharge into the environment. The ultimate fate and transport of the clay-bound molluscicides once discharged is unknown; many of these substances are nonbiodegradable and stay in the ecosystem long after discharge.
A common approach to mussel control is aqueous applications of chemicals such as chlorine for enclosed systems such as pipes and bays. Chlorine-based methods using hypochlorite, chlorine gas and chlorine dioxide necessarily involve careful practices to ensure that the chemicals are safely stored, and that employees handling the chemicals are not exposed to hazards and unnecessary risk. In addition, chlorine and other oxidizing chemicals are corrosive to equipment. Chemical treatments are toxic to other aquatic organisms and because of this non-targeted toxicity, facilities using chlorine and other chemical- based molluscicides may be required to deactivate or detoxify the treated water before discharge to meet environmental requirements (NETL 2006). Bisulfate or similar salts are used to help prevent the release of chlorine into the environment and reduce the impact on other aquatic organisms, contributing to salt loading in water bodies. Many molluscicides require the addition of clay to a treated water system to quench or deactivate the chemicals’ toxicity before discharge into the environment. The ultimate fate and transport of the clay-bound molluscicides once discharged is unknown; many of these substances are nonbiodegradable and stay in the ecosystem long after discharge.
An additional disadvantage of using chlorine is that the mussels perceive the chlorinated water as a threat, causing them to shut their valves for so long that very long application times are necessary to achieve results. The formation of harmful by-products is yet another area of concern; when chlorine combines with organic compounds in water, potentially carcinogenic substances such as trihalomethanes, haloacetic acids and dioxins are formed (U.S. Environmental Protection Agency [EPA] 1999; Thornton 2000).
A Biological Breakthrough
 The need for a new control method drove extensive research that led to an industry-changing discovery. Faced with the threat of zebra mussels fouling electric power facilities within New York State, a research consortium of New York State’s electric power generation companies contracted with New York State Museum Field Research Laboratory in 1991 for the screening of bacteria as potential biological control agents. The use of microbial, natural product compounds already had a clear record of commercial success and environmental safety in the control of invertebrate pests in North America, as well as globally (Rodgers 1993). Extensive laboratory screening trials of more than 700 bacterial strains identified a North American isolate, strain CL145A of Pseudomonas fluorescens, to be lethal to zebra and quagga mussels (Molloy 2002). Pseudomonas fluorescens is worldwide in distribution and is present in all North American water bodies. In nature, it is a harmless bacterial species that is found protecting the roots of plants from diseases.
The need for a new control method drove extensive research that led to an industry-changing discovery. Faced with the threat of zebra mussels fouling electric power facilities within New York State, a research consortium of New York State’s electric power generation companies contracted with New York State Museum Field Research Laboratory in 1991 for the screening of bacteria as potential biological control agents. The use of microbial, natural product compounds already had a clear record of commercial success and environmental safety in the control of invertebrate pests in North America, as well as globally (Rodgers 1993). Extensive laboratory screening trials of more than 700 bacterial strains identified a North American isolate, strain CL145A of Pseudomonas fluorescens, to be lethal to zebra and quagga mussels (Molloy 2002). Pseudomonas fluorescens is worldwide in distribution and is present in all North American water bodies. In nature, it is a harmless bacterial species that is found protecting the roots of plants from diseases.
Bringing the Solution to Market
In 2007 Marrone Bio Innovations (MBI) entered into a commercial partnership with the New York State Museum to bring this naturally occurring soil microorganism to market for the control of zebra and quagga mussels. The result was Zequanox—the industry’s first environmentally compatible molluscicide.The EPA registered Zequanox on July 29, 2011 and it is now registered in all states except Hawaii and In Canada for pipe use. Beginning in 2009, MBI in cooperation with the U.S. Bureau of Reclamation (Reclamation) conducted field trials of Zequanox under a Cooperative Research and Development Agreement (CRADA). The product was tested at Reclamation’s Davis Dam on the lower Colorado River, where supply lines were heavily infested. MBI also teamed with Ontario Power Generation of Ontario, Canada, to perform testing at the DeCew II Generating Station Facility. Ontario Power Generation, which had a 20-year history of chlorine control that had reached its maximum optimization potential and wanted to help bring a more sustainable mussel control solution to the market, assisted MBI in its commercial development of Zequanox (Van Oostrom, Peterson-Murray and Dow 2010).

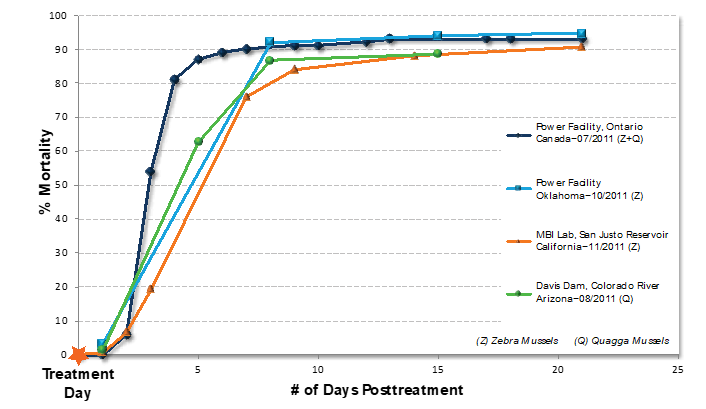
In 2011, MBI conducted a number of demonstration and full-scale Zequanox treatments throughout North America. The chart below summarizes test results of adult mussel treatments—conducted in different locations with varying water qualities—on both zebra and quagga mussels. Typical treatments
ranged from six to eight hours and mortality was scored from two weeks up to one month posttreatment. The following chart summarizes the early work in power and San Justo reservoir. Since these initial studies, several more facilities have been successfully treated such as Hoover Dam, First Light & Power, NRG, OGE, two drinking water plants (one in Mexico and one in Ireland), and others.
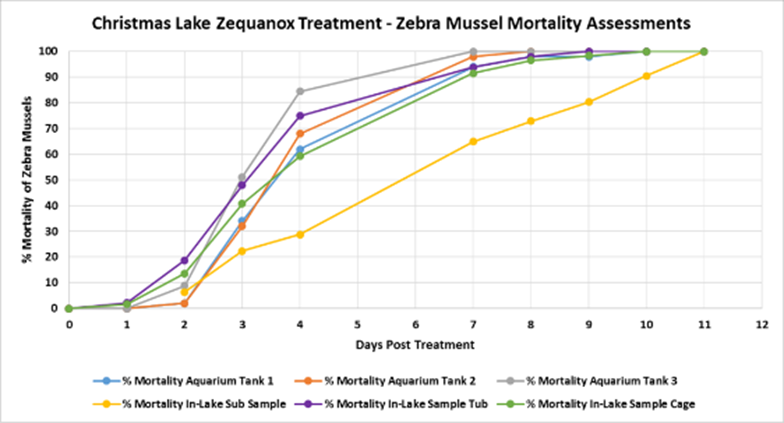
In addition, several lake treatments have been conducted including an eradication in Christmas Lake, MN.
How Does Zequanox Work?
Zequanox is composed of dead cells of the Pseudomonas fluorescens microorganism. The cells contain natural compounds that, when ingested, are lethal to zebra and quagga mussels during all life stages (veliger to adult). The primary natural product compound identified as causing the efficacy is a protein called FitD produced in the bacterial cells. It is patented by MBI and exclusively licensed to ISC. ISC has cloned this protein and developed a method to determine the protein levels in manufactured cells so we can ensure the product is efficacious from the fermentation factory.In the past, the only method to determine efficacy was a live mussel assay in the laboratory, which is very cumbersome. Now we can make product improvements more quickly using a gel method to detect the protein rather than using the mussel bioassay. The protein binds to the mussels’ gut cells and causes hemorrhaging. The mussels stop feeding and die.
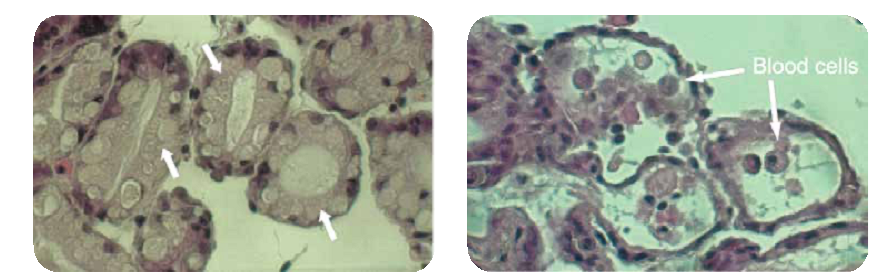
Extensive Toxicology Studies Demonstrate Selectivity
As previously noted, P. fluorescens is worldwide in distribution and is present in all North American water bodies. In nature it is a harmless bacterial species. Extensive toxicology studies have been conducted with Zequanox and the findings demonstrate that, unlike chemical molluscicides, Zequanox is highly selective toward zebra and quagga mussels and is of inherently low toxicity to non-target organisms (including other mussel species).A number of fish species from representative taxonomic groups have been tested. Several high-dose, multi-day toxicity studies on either Brown or Rainbow trout, which are often noted to be the most sensitive species in ecotoxicological studies, indicate minimal toxicity to trout. The results of studies on more hardy species—the fathead minnow of the cyprinid family, striped bass, and suckers—also show that the fish would be safe at the concentrations and exposure durations that they would experience in water bodies near facilities undergoing Zequanox treatments. Considering the relatively short treatment times using Zequanox, the immediate dilution in receiving waters and the rapid environmental breakdown of the product, no toxic effects to non-target fish species are expected or likely. This has been confirmed in open water treatments such as the Sleeping Bear Lake Michigan demo.
In addition to fish species, a broad range of invertebrate taxa has also been tested. No effects were noted on Daphnia (a common, small, free-swimming crustacean) or the sediment-dwelling amphipod crustacean Hyalella azteca. Based on the results of studies conducted on two benthic insects—mayfly nymphs and chironomids—no effects would be expected on free-swimming and benthic invertebrates. A 14-day study conducted with the mallard duck (a common, representative aquatic avian species) showed no mortality, no clinical signs of toxicity, no effect on body weight or feed consumption, and no pathological findings in all cases and at all concentrations tested. It is expected that exposure to water containing maximum treatment concentrations would not pose a threat to aquatic birds. Six common species of aquatic plants (common water plantain, small-flower umbrella sedge, nightshade, bindweed, mallow and curly dock) were immersed in water containing Zequanox at a concentration higher than that typically used for treatments. After six days of immersion, no signs of phytotoxicity were observed in any of the plants.
A range of freshwater mussel species (in the unionid family) was exposed to the maximum Zequanox concentrations. There was a complete absence of mortality in all cases, while in the same studies mortality in the zebra and quagga mussels consistently approached 100%. In addition, no mortality was observed in the native freshwater anadonta mussel. Based on these studies, no risks to native mussels are expected.
How Does Zequanox Compare With Alternative Solutions?
 Zequanox offers several advantages over chlorine, copper and other chemical pesticides, including safety, flexibility and ease of use. First and foremost, Zequanox poses very limited to no risk to workers, non-target species and the environment. As a reduced-risk pesticide, Zequanox is safe to store, handle and apply; only minimal personal protective equipment is needed. In contrast, chlorine, copper and other chemical pesticides are toxic to aquatic life and the environment (i.e., they often fall into the level 1 pesticide, or other high- risk category). These products require special handling, safety warning placards, sophisticated permitting, tracking and monitoring. If not properly managed, chlorine and other hazardous chemicals can cause serious (even fatal) harm to humans, and can cause irreparable harm to the environment. And as mentioned previously, the corrosive nature of oxidizing chemicals can limit the life span of valuable equipment or create the need for additional maintenance. To comply with the National Pollutant Discharge Elimination System, chlorine and harmful chemicals require special permitting, tracking, monitoring and detoxifying before discharge. The use of Zequanox carries none of these requirements, and detoxification is not required before discharge of the treated water.
Zequanox offers several advantages over chlorine, copper and other chemical pesticides, including safety, flexibility and ease of use. First and foremost, Zequanox poses very limited to no risk to workers, non-target species and the environment. As a reduced-risk pesticide, Zequanox is safe to store, handle and apply; only minimal personal protective equipment is needed. In contrast, chlorine, copper and other chemical pesticides are toxic to aquatic life and the environment (i.e., they often fall into the level 1 pesticide, or other high- risk category). These products require special handling, safety warning placards, sophisticated permitting, tracking and monitoring. If not properly managed, chlorine and other hazardous chemicals can cause serious (even fatal) harm to humans, and can cause irreparable harm to the environment. And as mentioned previously, the corrosive nature of oxidizing chemicals can limit the life span of valuable equipment or create the need for additional maintenance. To comply with the National Pollutant Discharge Elimination System, chlorine and harmful chemicals require special permitting, tracking, monitoring and detoxifying before discharge. The use of Zequanox carries none of these requirements, and detoxification is not required before discharge of the treated water.
Applications of Zequanox are less labor intensive and less operationally disruptive than chemical methods. Zequanox treatments can be done during normal facility operations and typically occur within a six- to eight-hour period. This timeframe is in contrast to chlorine treatments, which can require several weeks of around-the-clock treatment, and often require special procedures to ensure worker safety during the treatments.
Zequanox offers additional flexibility in that it is proven effective in a broader range of water conditions and temperatures than chlorine, thus expanding the “treatment season” during which Zequanox treatments can be effective. Zequanox also offers a number of advantages when compared with UV and microfiltration solutions. First, Zequanox can be applied using standard injection equipment, so facility operators can implement a Zequanox control program quickly and easily. Zequanox can be employed without having to undergo an arduous capital budgeting process and equipment installation, and without incurring the additional overhead of ongoing equipment maintenance. The aqueous formulation of Zequanox provides the added benefit of being able to reach and treat even the smallest of crevices in the water system, whereas mechanical solutions offer control only at a fixed location.
Zequanox also offers the unmatched ability to tailor the treatment regimen to achieve the desired balance of mussel control, application frequency and shell debris management.
Since Zequanox was developed, copper has gained usage for controlling zebra and quagga mussels in open waters, particularly Earthtec QZ. It is an effective product, however it is less environmentally friendly and more toxic to non-target organisms than Zequanox. For humans, Zequanox has a CAUTION label – the safest category, whereas copper is WARNING, a higher risk category. Also, there are warnings about non-target organisms on the Earthtec QZ EPA label. “This pesticide is toxic to fish and aquatic invertebrates.” “Waters treated with this product may be hazardous to aquatic organisms.” This is not the case with Zequanox, which has similarly high efficacy as copper.
In addition, Zequanox can mesh with sustainability initiatives. Because Zequanox is fermented in large stainless steel vessels instead of made synthetically or mined, it has a lower carbon footprint and is less fossil fuel intensive than competing products. Including Zequanox’s high safety to humans and nontarget organisms, it makes it the product of choice for increasing sustainability and environmental compliance initiatives.
A Comparison of Zebra and Quagga Mussel Control Methods
| Chlorine & Other Chemical Pesticides | Microfiltration/UV | Copper | Zequanox | |
|---|---|---|---|---|
| Application Time | Days to weeks | Continuous | 24 hours to several days | 6 hours |
| Startup Investment | Medium | High | Medium | Limited |
| Worker Safety Requirements | High | Minimal | Medium | Minimal |
| PPE Requirements | High | Minimal | High | Minimal |
| Discharge Requirements | Detoxification may be required | None | Some restrictions for potable water | None |
| Environmental & Nontarget Risk | Highly toxic to most organisms; Forms toxic byproducts | None | EPA classifies copper as an environmental pollutant; Low doses may have lower effect but cannot be used at pHs below 5.5 & low DO; Specific fish restrictions for lake treatments | None |
| Equipment Corrosion Risk | High | None | Cannot use steel, nylon, brass or copper containers/pipes | None |
| Water Temp & Water Quality Effects on Control | Limited efficacy below 8C; lower efficacy when organic matter & algae are present | Efficacy comprised in cloudy waters with organic matter, & algae infested waters | Minimal; Lower temps may require longer treatment times | Minimal water quality effects. Effective down to 8C |
| Regulatory Restrictions | High. May require state permits | NA | Some. May require state permits | Low |
Zequanox Second Generation Product Under Development
Because Zequanox is fermented in large stainless steel vessels instead of made synthetically or mined, it has a lower carbon footprint and less fossil fuel intensive than competing products. However, fermentation is generally more expensive than synthesis. As such, it has been more expensive than chemicals on a line item basis (not including all the permitting, safety requirements, corrosion, etc. for chemicals). ISC is working on enhancing the fermentation manufacturing process and formulation to drive down the cost to cement it as the product of choice. ISC has a CRADA with the USGS to develop and test a new formulation that sinks to the bottom of a water body where the mussels live. This formulation will eliminate the need for barriers and curtains required with the current wettable powder product for large bodies of water.
Conclusion
Throughout North America and Europe, zebra and quagga mussels have seriously affected industrial and commercial operations by restricting water flow in heat exchangers, condensers, fire suppression systems, and service and cooling water systems, as well as by damaging other infrastructure and equipment. In addition, they continue to spread into lakes and rivers, including tributaries to sensitive salmon habitats. Just recently (July 2024), the mussels were reported in new areas of the Colorado River, a lake in Minnesota and Manitoba Canada.
The first and only biological mussel control solution, Zequanox offers what no other mussel control solution does—a highly effective, flexible method that requires little or no capital investment and that can be used without putting employees or the environment at risk from harsh chemicals. Using Zequanox for invasive mussel control allows facility owners to support environmental stewardship while protecting operations and assets. In addition, Zequanox offers water resource managers a very effective but safer and more environmentally friendly solution for rapid response to eradicate new infestations as well as treatment of established infestations.
Learn More: Zequanox Product Page
REFERENCES Benson, A. J. and D. Raikow. 2011. Dreissena polymorpha. USGS Nonindigenous Aquatic Species Database, Gainesville, FL. http://nas.er.usgs.gov/queries/FactSheet.aspx?speciesID=5. Revision Date: July 8, 2010. Accessed January 3, 2012. Benson, A. J., M. M. Richerson, E. Maynard, J. Larson, and A. Fusaro. 2012. Dreissena bugensis. USGS Nonindigenous Aquatic Species Database, Gainesville, FL. http://nas.er.usgs.gov/queries/FactSheet.aspx?speciesID=95. Revision Date: March 19, 2012. Accessed May 29, 2012. Angelique D. Dahlberg, Diane L. Waller, David Hammond, Keegan Lund, and Nicholas B. D. Phelps. Sci Rep. 2023; 13: 10410. Open water dreissenid mussel control projects: lessons learned from a retrospective analysis. Published online 2023 Jun 27. doi: 10.1038/s41598-023-36522-5 Keegan Lund, Kylie Bloodsworth Cattoor, Eric Fieldseth, Jill Sweet & Michael A. McCartney (2018) Zebra mussel (Dreissena polymorpha) eradication efforts in Christmas Lake, Minnesota, Lake and Reservoir Management, 34:1, 7-20, DOI: 10.1080/10402381.2017.1360417 Molloy, D. P. 1991. Biological control of zebra mussels: Use of parasites and toxic microorganisms. Journal of Shellfish Research 10:260. Molloy, D. P. 1998. The potential for using biological control technologies in the management of Dreissena spp. Journal of Shellfish Research 17:177-183. Molloy, D. P. 2001. A Method for controlling Dreissena species. US Patent and Trademark Office, US Department of Commerce. Patent No. 6,194,194, filed December 17, 1997 and issued February 27, 2001. Molloy, D. P. 2002. Biological control of zebra mussels. Proceedings of the Third California Conference on Biological Control. University of California, Davis. pp. 86–94. Rodgers, P. B. 1993. Potential of biopesticides in agriculture. Pesticide Science 39:117-129. Thornton, J. 2000. Pandora’s Poison: Chlorine, Health, and a New Environmental Strategy. MIT Press, Cambridge, Massachusetts. U.S. Department of Energy, National Energy Technology Laboratory (NETL). 2006. Effectiveness of a Microbial Control Agent Method of Controlling Zebra Mussel Fouling Compared to Chlorine Injection. Draft Report. Prepared by WorleyParsons Group, Inc. WorleyParsons Report No. EJ-2004-06. February 17, 2006. U.S. Department of the Interior, United States Geological Survey (USGS). 2008. Invasive Invertebrates: Zebra Mussel. http://www.glsc.usgs.gov/main.php?content=research_invasive_zebramussel&title=Invasive%20Invertebrates0&menu=research_invasive_i. Revision Date January 31, 2008. Accessed December 30, 2011. USGS. 2011. Zebra Mussels Cause Economic and Ecological Problems in the Great Lakes. Great Lakes Science Center Fact Sheet 2000-6. July 2011. http://www.glsc.usgs.gov/_files/factsheets/2000-6%20Zebra%20Mussels.pdf. Accessed December 12, 2011. U.S. Environmental Protection Agency (EPA). 1999. Wastewater technology fact sheet: Chlorine disinfection. U.S. Environmental Protection Agency, Washington, DC. EPA/832-F99-062. U.S. Army Corps of Engineers. 2002. Zebra Mussel Information System: Impacts. http://el.erdc.usace.army.mil/ zebra/zmis/. Accessed May 29, 2012. Van Oostrom, Tony, Kelly Peterson-Murray, and Sarahann Dow. 2010. “Demonstration Trials at DeCew II Generating Station at Ontario Power Generation Using Zequanox.” Presented at the International Conference on Aquatic Invasive Species, San Diego, California, August 29–September 2, 2010. Gregory W. Whitledge, Megan M. Weber, Jessi DeMartini, John Oldenburg, Dave Roberts, Carolyn Link, Sarahann M. Rackl, Neil P. Rude, Andrew J. Yung, Lindsey R. Bock and Devon C. Oliver. An evaluation Zequanox® efficacy and application strategies for targeted control of zebra mussels in shallow-water habitats in lakes. Management of Biological Invasions (2015) Volume 6, 71-82, 2014.
